Medical Research Productsfrom ECO MEDICS
Medical Research Products
from ECO MEDICS
Medical Research Products
from ECO MEDICS
ECO MEDICS Product Overview view 440KB PDF File
Pulmonary Function Test (PFT) & Lung Clearance Index (LCI) Measurements
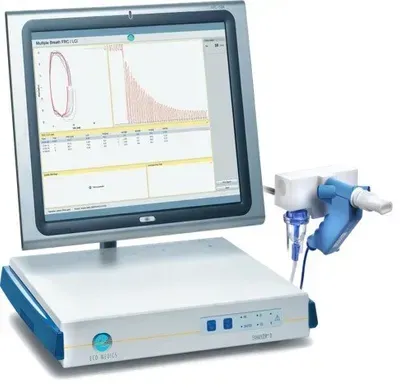
EXHALYZER D® with SPIROWARE® v3.3.2
view 2.8MB PDF file
Pulmonary function testing (PFT) for detection and monitoring of ventilation inhomogeneity and small airway diseases such as Asthma, cystic fibrosis (CF) and COPD research. The wide application range includes nitrogen wash out tests (single N2SBW & multiple N2MBW breath) for lung clearance index (LCI) determination and slope 3 analysis (SnIII), tidal breathing analysis (TBFVL), FRC, spirometry and capnography.
- Excellent accuracy: State of the art components ensure high precision and accuracy and are immune to turbulence, humidity, or temperature changes in the respiratory flow
- Automatic Quality Control: Measurements are constantly monitored to meet defined quality criteria
- One instrument for infants, children, and adults (>3kg body weight) facilitated by exchangeable dead space reducers
- Fully compliant to ATS/ERS guidelines for pulmonary function testing
- Easy to operate with SPIROWARE® software for analysis, evaluation, and documentation of measurements
- Fully upgradable: The modular setup allows individual upgrades to your needs
Exhaled Nitric Oxide (FeNO) and Nasal (nNO) Application Measurements
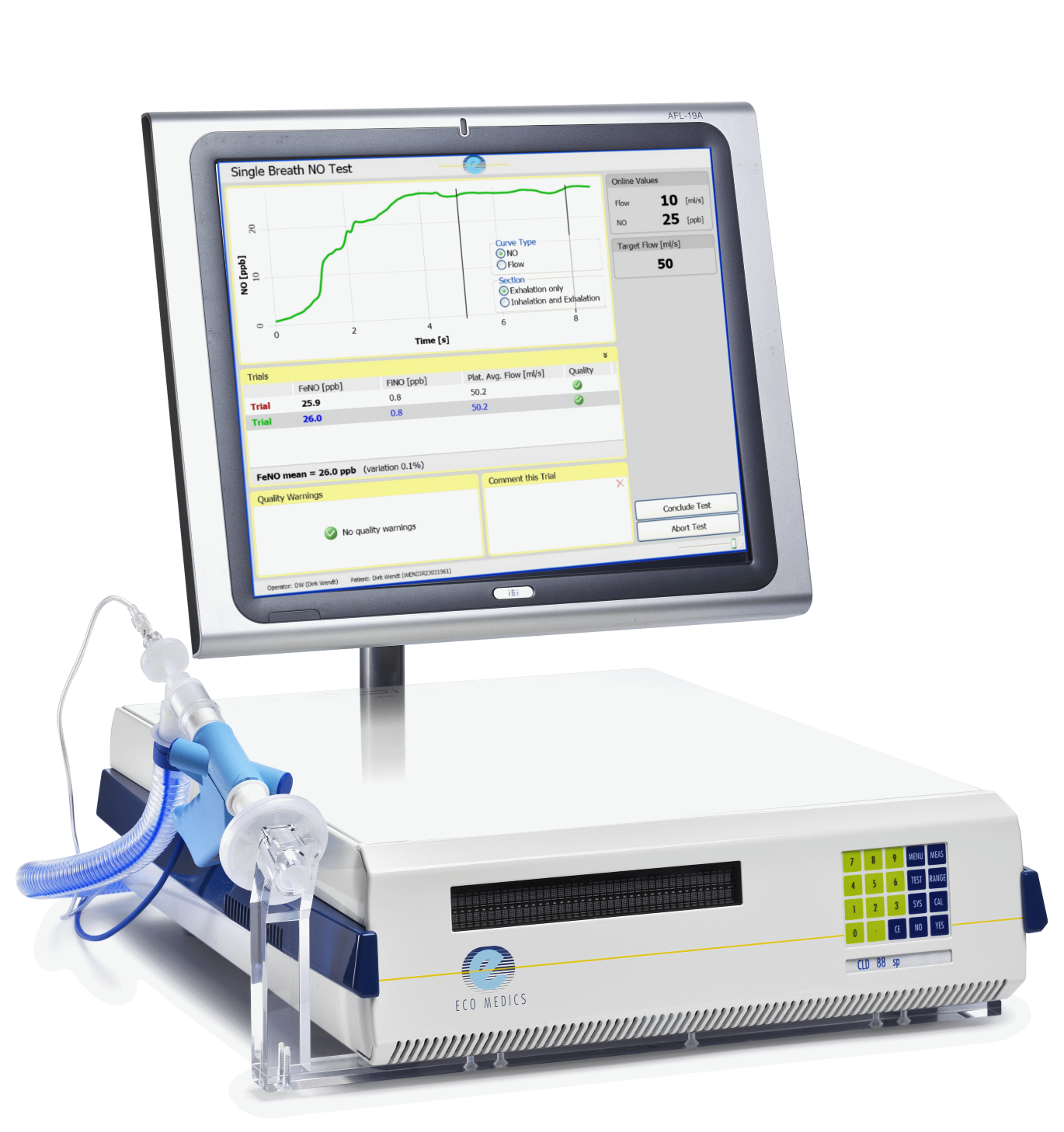
Analyzer CLD 88 sp for FeNO testing with SPIROWARE® 3.3.2
view 2.9MB PDF file
- Chemiluminescence nitric oxide gas analyzer
- 0-5,000 ppb NO range with 0.06 ppb detection level
- SPIROWARE® version 3.3.2 data acquisition and evaluation software for determination of FeNO production with incentive screen feedback for exhalation flow control
- Integrated oil-free diaphragm pump
- High sensitivity and reproducibility guarantee reliable results
- Wide application range: bronchial, alveolar, and nasal NO analysis, single and multiple breath analysis, offline NO analysis
- One instrument for infants, children, and adults (>3kg body weight)
- CE MDD approval for clinical use in Europe
- Fully compliant to ATS/ERS guidelines for FeNO analysis
- Easy to use with SPIROWARE® software for analysis, evaluation, and documentation of NO measurements
This video demonstrates step-by-step how to calibrate the ECO MEDICS ANALYZER CLD 88 Series. Calibration prior to FeNO measurement is important to enable efficient lung function measurement and to obtain clinically useful and reproducible data.
DENOX 88
view 242K PDF file
NO-free air supply and advanced adaptive flow control to support FeNO measurements in accordance with the ATS/ERS guideline.
- NO Free Air and Adaptive Flow Control for the CLD 88 sp
- Variable exhalation flow resistances
- NO-free air generation from ambient air, no gas supply required
- The Integrated adaptive flow control allows even pre-school kids to perform single breath tests following ATS/ERS recommendations
- Suitable for single and multiple breath tests from infants (>3 kg body weight) to adults
FLEXIBLE SOFTWARE PACKAGE
SPIROWARE® Data Acquisition and Evaluation Software v3.3.2
view 5.5MB PDF file
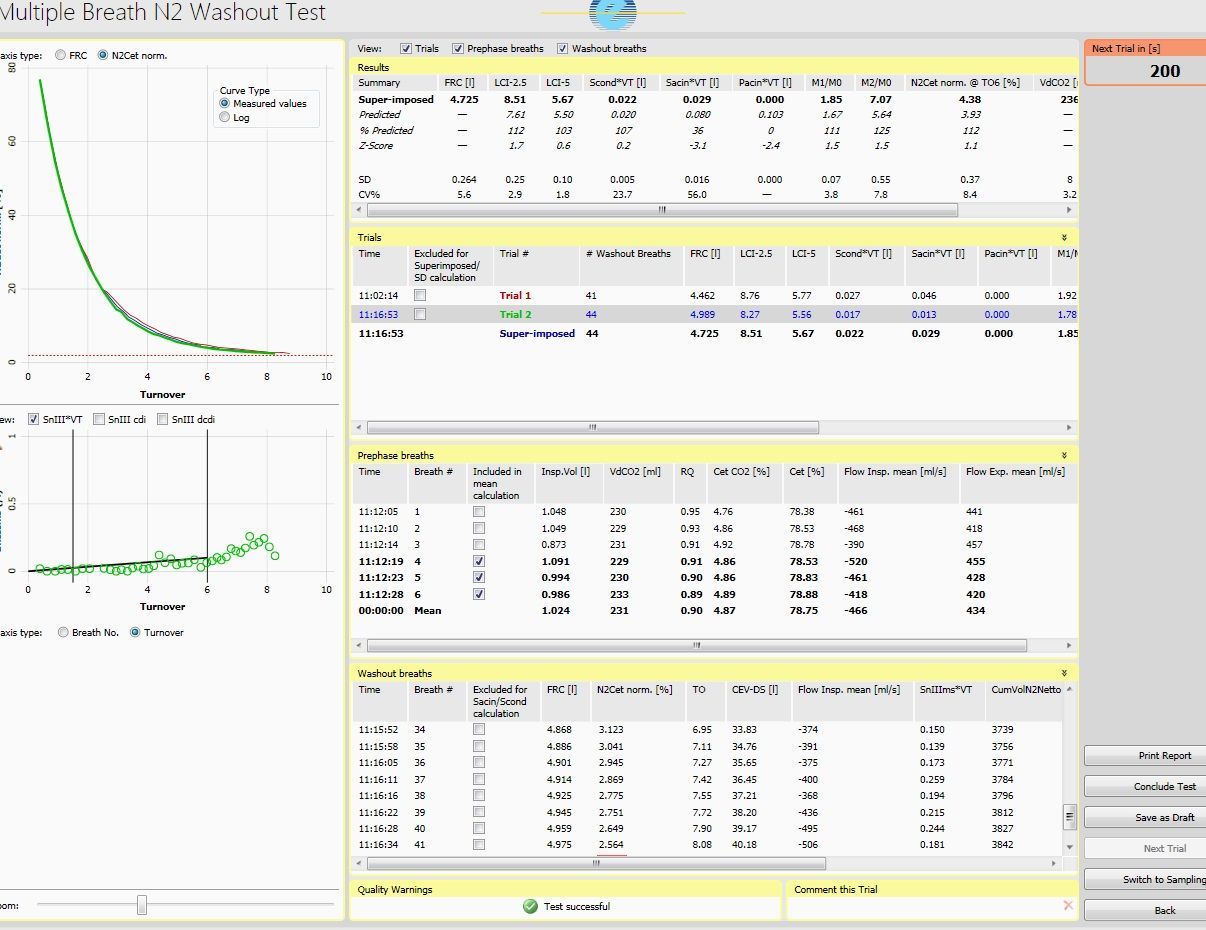

Easy to use and flexible software for performing, evaluating, and reporting comprehensive pulmonary data with the EXHALYZER® D or ANALYZER CLD 88 sp instruments.
- Intuitive graphical user interface for an efficient workflow
- Straightforward data evaluation, including trend charts and statistical analysis
- Reference database to assist result interpretation
- Excellent documentation options with a wide selection of pre-defined, editable reports
- Built-in quality control for monitoring the instrument and measurement
- Easy data exchange through HIS interface or export of raw data
Announcing SPIROWARE® v3.3.2
has been released!
Including:
Spirometry application for EXHALYZER® D and ANALYZER CLD 88 sp
(SW license required)
• Fully compliant with ATS/ERS 2019 standard
• Volumetric Capnography is included in all multiple breath washout tests
• Flow calibration: Differentiation between calibration and verification
• Calibration for Spirometry at different flows
• Better Usability: Reference models , medication definitions , comment
management
• EXHALYZER ® D hardware: new additional gas module ports
• New Report Templates
The ECO MEDICS products for exhaled FeNO (Fractional exhaled Nitric Oxide), Nasal NO and PFT/LCI measurements are not FDA approved, and are sold for basic physiological research only.
Offline FeNO measurements in accordance with ATS/ERS guideline.
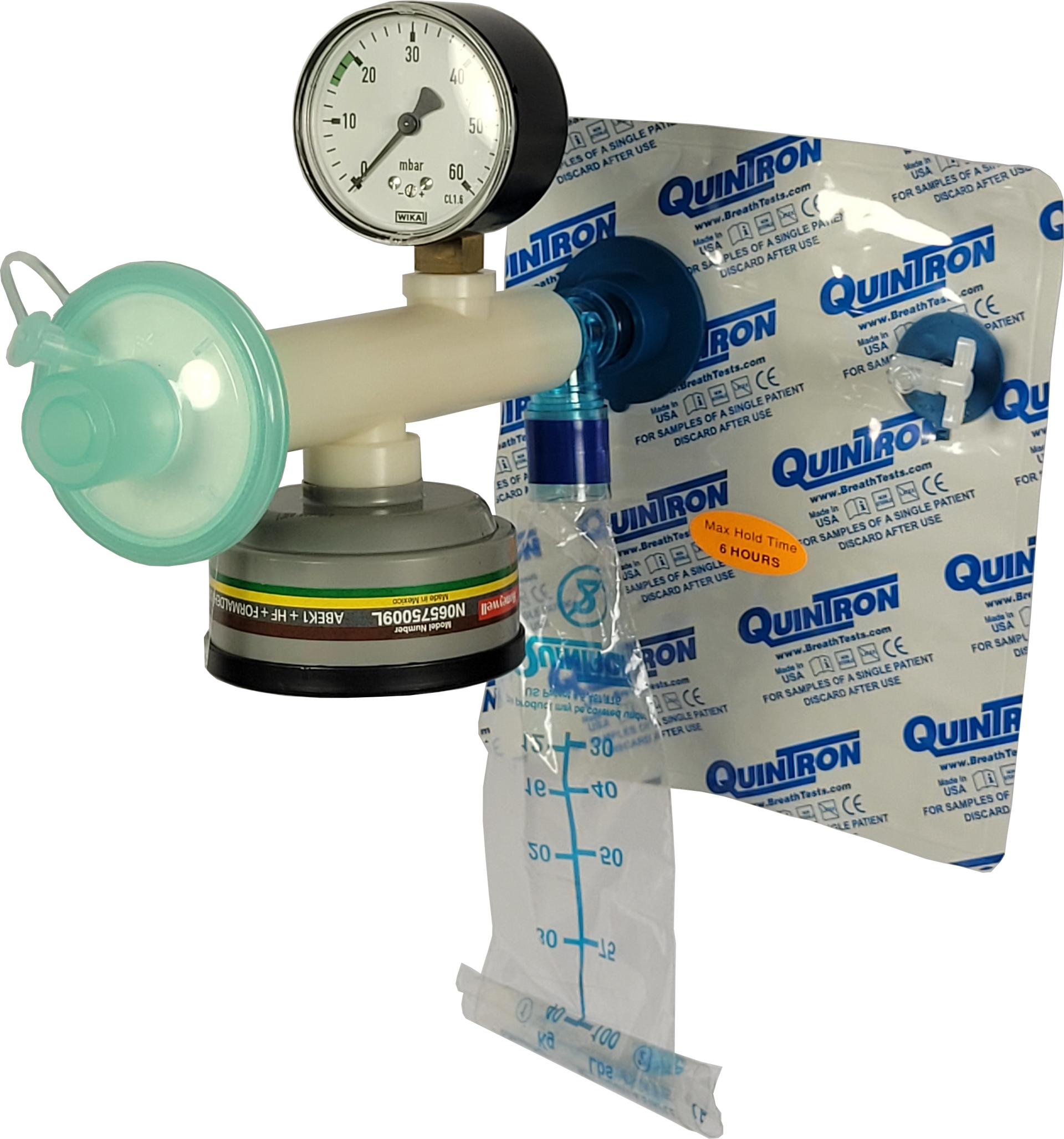
- Integration Software for ANALYZER CLD 88 sp
- Optional calculation of Alveolar NO concentration
- Suitable for remote NO collection sites
- More efficient use of FeNO ANALYZER CLD 88 sp
- Increased flexibility and independence of your FeNO measurements
Liquid NO Application view 344K PDF file
nCLD 88 with EDAQ PowerChrom
view 4.9 MB PDF file
- 0-5,000 ppb with 0.060 ppb detection level
- Chemiluminescence NO measurement
- Optional Molybdenum converter for NO2 determination
Pulmonary Function Test (PFT) & Lung Clearance Index (LCI) Measurements

EXHALYZER D® with SPIROWARE® v3.3.2
view 2.8MB PDF file
Pulmonary function testing (PFT) for detection and monitoring of ventilation inhomogeneity and small airway diseases such as Asthma, cystic fibrosis (CF) and COPD research. The wide application range includes nitrogen wash out tests (single N2SBW & multiple N2MBW breath) for lung clearance index (LCI) determination and slope 3 analysis (SnIII), tidal breathing analysis (TBFVL), FRC, spirometry and capnography.
- Excellent accuracy: State of the art components ensure high precision and accuracy and are immune to turbulence, humidity, or temperature changes in the respiratory flow
- Automatic Quality Control: Measurements are constantly monitored to meet defined quality criteria
- One instrument for infants, children, and adults (>3kg body weight) facilitated by exchangeable dead space reducers
- Fully compliant to ATS/ERS guidelines for pulmonary function testing
- Easy to operate with SPIROWARE® software for analysis, evaluation, and documentation of measurements
- Fully upgradable: The modular setup allows individual upgrades to your needs
Exhaled Nitric Oxide (FeNO) and Nasal (nNO) Application Measurements

Analyzer CLD 88 sp for FeNO testing with SPIROWARE® 3.3.2
view 2.9MB PDF file
- Chemiluminescence nitric oxide gas analyzer
- 0-5,000 ppb NO range with 0.06 ppb detection level
- SPIROWARE® version 3.3.2 data acquisition and evaluation software for determination of FeNO production with incentive screen feedback for exhalation flow control
- Integrated oil-free diaphragm pump
- High sensitivity and reproducibility guarantee reliable results
- Wide application range: bronchial, alveolar, and nasal NO analysis, single and multiple breath analysis, offline NO analysis
- One instrument for infants, children, and adults (>3kg body weight)
- CE MDD approval for clinical use in Europe
- Fully compliant to ATS/ERS guidelines for FeNO analysis
- Easy to use with SPIROWARE® software for analysis, evaluation, and documentation of NO measurements
This video demonstrates step-by-step how to calibrate the ECO MEDICS ANALYZER CLD 88 Series. Calibration prior to FeNO measurement is important to enable efficient lung function measurement and to obtain clinically useful and reproducible data.
DENOX 88
view 242K PDF file
NO-free air supply and advanced adaptive flow control to support FeNO measurements in accordance with the ATS/ERS guideline.
- NO Free Air and Adaptive Flow Control for the CLD 88 sp
- Variable exhalation flow resistances
- NO-free air generation from ambient air, no gas supply required
- The Integrated adaptive flow control allows even pre-school kids to perform single breath tests following ATS/ERS recommendations
- Suitable for single and multiple breath tests from infants (>3 kg body weight) to adults
FLEXIBLE SOFTWARE PACKAGE
SPIROWARE® Data Acquisition and Evaluation Software v3.3.2
view 5.5MB PDF file


Easy to use and flexible software for performing, evaluating, and reporting comprehensive pulmonary data with the EXHALYZER® D or ANALYZER CLD 88 sp instruments.
- Intuitive graphical user interface for an efficient workflow
- Straightforward data evaluation, including trend charts and statistical analysis
- Reference database to assist result interpretation
- Excellent documentation options with a wide selection of pre-defined, editable reports
- Built-in quality control for monitoring the instrument and measurement
- Easy data exchange through HIS interface or export of raw data
Announcing SPIROWARE® v3.3.2
has been released!
Including:
Spirometry application for EXHALYZER® D and ANALYZER CLD 88 sp
(SW license required)
• Fully compliant with ATS/ERS 2019 standard
• Volumetric Capnography is included in all multiple breath washout tests
• Flow calibration: Differentiation between calibration and verification
• Calibration for Spirometry at different flows
• Better Usability: Reference models , medication definitions , comment
management
• EXHALYZER ® D hardware: new additional gas module ports
• New Report Templates
The ECO MEDICS products for exhaled FeNO (Fractional exhaled Nitric Oxide), Nasal NO and PFT/LCI measurements are not FDA approved, and are sold for basic physiological research only.
Offline FeNO measurements in accordance with ATS/ERS guideline.

- Integration Software for ANALYZER CLD 88 sp
- Optional calculation of Alveolar NO concentration
- Suitable for remote NO collection sites
- More efficient use of FeNO ANALYZER CLD 88 sp
- Increased flexibility and independence of your FeNO measurements
Liquid NO Application view 344K PDF file
nCLD 88 with EDAQ PowerChrom
view 4.9 MB PDF file
- 0-5,000 ppb with 0.060 ppb detection level
- Chemiluminescence NO measurement
- Optional Molybdenum converter for NO2 determination
ECO MEDICS Product Overview view 440KB PDF File
Pulmonary Function Test (PFT) & Lung Clearance Index (LCI) Measurements

EXHALYZER D® with SPIROWARE® v3.3.2
view 2.8MB PDF file
Pulmonary function testing (PFT) for detection and monitoring of ventilation inhomogeneity and small airway diseases such as Asthma, cystic fibrosis (CF) and COPD research. The wide application range includes nitrogen wash out tests (single N2SBW & multiple N2MBW breath) for lung clearance index (LCI) determination and slope 3 analysis (SnIII), tidal breathing analysis (TBFVL), FRC, spirometry and capnography.
- Excellent accuracy: State of the art components ensure high precision and accuracy and are immune to turbulence, humidity, or temperature changes in the respiratory flow
- Automatic Quality Control: Measurements are constantly monitored to meet defined quality criteria
- One instrument for infants, children, and adults (>3kg body weight) facilitated by exchangeable dead space reducers
- Fully compliant to ATS/ERS guidelines for pulmonary function testing
- Easy to operate with SPIROWARE® software for analysis, evaluation, and documentation of measurements
- Fully upgradable: The modular setup allows individual upgrades to your needs
Exhaled Nitric Oxide (FeNO) and Nasal (nNO) Application Measurements

Analyzer CLD 88 sp for FeNO testing with SPIROWARE® 3.3.2
view 2.9MB PDF file
- Chemiluminescence nitric oxide gas analyzer
- 0-5,000 ppb NO range with 0.06 ppb detection level
- SPIROWARE® version 3.3.2 data acquisition and evaluation software for determination of FeNO production with incentive screen feedback for exhalation flow control
- Integrated oil-free diaphragm pump
- High sensitivity and reproducibility guarantee reliable results
- Wide application range: bronchial, alveolar, and nasal NO analysis, single and multiple breath analysis, offline NO analysis
- One instrument for infants, children, and adults (>3kg body weight)
- CE MDD approval for clinical use in Europe
- Fully compliant to ATS/ERS guidelines for FeNO analysis
- Easy to use with SPIROWARE® software for analysis, evaluation, and documentation of NO measurements
This video demonstrates step-by-step how to calibrate the ECO MEDICS ANALYZER CLD 88 Series. Calibration prior to FeNO measurement is important to enable efficient lung function measurement and to obtain clinically useful and reproducible data.
DENOX 88
view 242K PDF file
NO-free air supply and advanced adaptive flow control to support FeNO measurements in accordance with the ATS/ERS guideline.
- NO Free Air and Adaptive Flow Control for the CLD 88 sp
- Variable exhalation flow resistances
- NO-free air generation from ambient air, no gas supply required
- The Integrated adaptive flow control allows even pre-school kids to perform single breath tests following ATS/ERS recommendations
- Suitable for single and multiple breath tests from infants (>3 kg body weight) to adults
FLEXIBLE SOFTWARE PACKAGE
SPIROWARE® Data Acquisition and Evaluation Software v3.3.2
view 5.5MB PDF file


Easy to use and flexible software for performing, evaluating, and reporting comprehensive pulmonary data with the EXHALYZER® D or ANALYZER CLD 88 sp instruments.
- Intuitive graphical user interface for an efficient workflow
- Straightforward data evaluation, including trend charts and statistical analysis
- Reference database to assist result interpretation
- Excellent documentation options with a wide selection of pre-defined, editable reports
- Built-in quality control for monitoring the instrument and measurement
- Easy data exchange through HIS interface or export of raw data
Announcing SPIROWARE® v3.3.2
has been released!
Including:
Spirometry application for EXHALYZER® D and ANALYZER CLD 88 sp
(SW license required)
• Fully compliant with ATS/ERS 2019 standard
• Volumetric Capnography is included in all multiple breath washout tests
• Flow calibration: Differentiation between calibration and verification
• Calibration for Spirometry at different flows
• Better Usability: Reference models , medication definitions , comment
management
• EXHALYZER ® D hardware: new additional gas module ports
• New Report Templates
The ECO MEDICS products for exhaled FeNO (Fractional exhaled Nitric Oxide), Nasal NO and PFT/LCI measurements are not FDA approved, and are sold for basic physiological research only.
Offline FeNO measurements in accordance with ATS/ERS guideline.

- Integration Software for ANALYZER CLD 88 sp
- Optional calculation of Alveolar NO concentration
- Suitable for remote NO collection sites
- More efficient use of FeNO ANALYZER CLD 88 sp
- Increased flexibility and independence of your FeNO measurements
Liquid NO Application view 344K PDF file
nCLD 88 with EDAQ PowerChrom
view 4.9 MB PDF file
- 0-5,000 ppb with 0.060 ppb detection level
- Chemiluminescence NO measurement
- Optional Molybdenum converter for NO2 determination




